Home » Projects » Genetic Stock Improvement of Hard Clams Projects » Increasing Hard Clam Production by Using Biomarkers of Thermal Tolerance
Home » Projects » Genetic Stock Improvement of Hard Clams Projects » Increasing Hard Clam Production by Using Biomarkers of Thermal Tolerance
 subtropical temperatures allow for a long growing season. However, growers across Florida have experienced chronic losses of market-size clams when summer water temperatures exceed 90oF. Climate change will certainly have an effect on worldwide agriculture; crops that are currently near climate thresholds, such as clams, are likely to suffer. There is a need for a heat-tolerant clam strain if the Florida industry is to reduce current summer mortalities and adapt to future climate change. In previous breeding studies (triploidy, hybridization), the project team demonstrated that thermal tolerance in clams is under genetic control. In both projects, we produced families from single-parent crosses, with parents selected at random from available broodstock. Some families consistently performed better than others. Our work suggests that heat shock proteins may be the mechanism whereby different strains of clams tolerate high temperatures. Heat-shock proteins (Hsp) are involved in the formation, transportation, and degradation of proteins. Some Hsp increase when cells are exposed to elevated temperatures, or other stressors that damage proteins, and are referred to as inducible. Other forms of Hsp are synthesized under non-stressful conditions for cellular housekeeping and are referred to as cognates. In one project, we found that a hard clam family having approximately twice as much cognate Hsp compared to two other families, also had significantly greater survival (93% compared to 28% and 39%) after heat challenge. In addition, other studies indicate that changes in metabolic rate in response to thermal stress may play a role in survival and could also be heritable. Together, these data suggest that using biomarkers of thermo-tolerance, such as Hsp, we can target particular genetically distinct groups for selective breeding, thus reducing the time and resources needed for strain development.
subtropical temperatures allow for a long growing season. However, growers across Florida have experienced chronic losses of market-size clams when summer water temperatures exceed 90oF. Climate change will certainly have an effect on worldwide agriculture; crops that are currently near climate thresholds, such as clams, are likely to suffer. There is a need for a heat-tolerant clam strain if the Florida industry is to reduce current summer mortalities and adapt to future climate change. In previous breeding studies (triploidy, hybridization), the project team demonstrated that thermal tolerance in clams is under genetic control. In both projects, we produced families from single-parent crosses, with parents selected at random from available broodstock. Some families consistently performed better than others. Our work suggests that heat shock proteins may be the mechanism whereby different strains of clams tolerate high temperatures. Heat-shock proteins (Hsp) are involved in the formation, transportation, and degradation of proteins. Some Hsp increase when cells are exposed to elevated temperatures, or other stressors that damage proteins, and are referred to as inducible. Other forms of Hsp are synthesized under non-stressful conditions for cellular housekeeping and are referred to as cognates. In one project, we found that a hard clam family having approximately twice as much cognate Hsp compared to two other families, also had significantly greater survival (93% compared to 28% and 39%) after heat challenge. In addition, other studies indicate that changes in metabolic rate in response to thermal stress may play a role in survival and could also be heritable. Together, these data suggest that using biomarkers of thermo-tolerance, such as Hsp, we can target particular genetically distinct groups for selective breeding, thus reducing the time and resources needed for strain development.
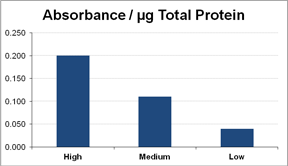 Adult hard clams were collected between October and December 2010. Potential broodstock (n = 550 total from 11 groups) were sampled for measurements of cognate Hsp70 concentration during January through April of 2011. We found that relative levels of cognate Hsp70 varied significantly among individual adult clams within the examined geographic or cultured groups and could be categorized as high, medium, and low (Figure). Therefore, we were able to select high- and low-cognate Hsp70 expressing broodstock for breeding; on average, the putative high Hsp70 broodstock had four times as much relative Hsp70 (based on absorbance value) compared to the low Hsp70 parents. Three families were produced from single-parent crosses of high-expressing parental stock and three families were produced from single-parent crosses of low-expressing parental stock in May 2011. Egg numbers ranged from 0.96-2.2 million and survival of larvae from day 2 to day 7 ranged from 43-84% for the low-expressing group and 78-98% for the high-expressing group. In September 2011, seed were transferred to field nursery systems on a commercial lease in the Indian River Lagoon located near Sebastian; triplicate bags of 8,000 seed were planted for each of the six families. Production of these families occurred too late to conduct the field nursery and subsequent growout and harvest during the natural heat stress of summer. Therefore, a second production spawn of putative high- and low-Hsp70 families was conducted in December 2011 and field nursed on an experimental lease in the Gulf of Mexico near Cedar Key during the summer of 2012. Final harvest of both replicate sets of hard clam families will occur in 2013. If Hsp levels in progeny are correlated with parental Hsp levels and high-Hsp families exhibit higher survival in the field and laboratory challenges, Hsp may be considered a biomarker for selective breeding of heat-tolerant hard clams.
A presentation on this project was made at an industry meeting and can be viewed below in PDF format.
Adult hard clams were collected between October and December 2010. Potential broodstock (n = 550 total from 11 groups) were sampled for measurements of cognate Hsp70 concentration during January through April of 2011. We found that relative levels of cognate Hsp70 varied significantly among individual adult clams within the examined geographic or cultured groups and could be categorized as high, medium, and low (Figure). Therefore, we were able to select high- and low-cognate Hsp70 expressing broodstock for breeding; on average, the putative high Hsp70 broodstock had four times as much relative Hsp70 (based on absorbance value) compared to the low Hsp70 parents. Three families were produced from single-parent crosses of high-expressing parental stock and three families were produced from single-parent crosses of low-expressing parental stock in May 2011. Egg numbers ranged from 0.96-2.2 million and survival of larvae from day 2 to day 7 ranged from 43-84% for the low-expressing group and 78-98% for the high-expressing group. In September 2011, seed were transferred to field nursery systems on a commercial lease in the Indian River Lagoon located near Sebastian; triplicate bags of 8,000 seed were planted for each of the six families. Production of these families occurred too late to conduct the field nursery and subsequent growout and harvest during the natural heat stress of summer. Therefore, a second production spawn of putative high- and low-Hsp70 families was conducted in December 2011 and field nursed on an experimental lease in the Gulf of Mexico near Cedar Key during the summer of 2012. Final harvest of both replicate sets of hard clam families will occur in 2013. If Hsp levels in progeny are correlated with parental Hsp levels and high-Hsp families exhibit higher survival in the field and laboratory challenges, Hsp may be considered a biomarker for selective breeding of heat-tolerant hard clams.
A presentation on this project was made at an industry meeting and can be viewed below in PDF format.